 Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). Temperature given as °C, °F, K and °R.
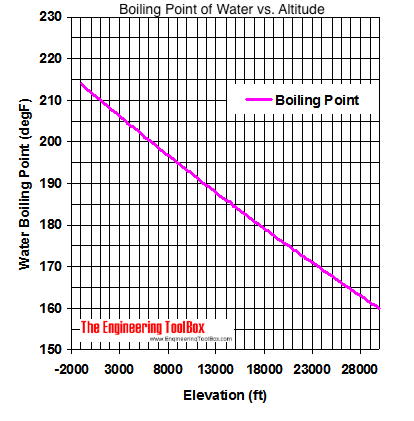
Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). Temperature given as °C, °F, K and °R. But, the boiling point of water changes with elevation. The boiling point is a higher temperature below sea level and a lower temperature above sea level. The normal boiling point of water is 100°C (212°F, 373.1 K) at sea level. Boiling occurs when vapor pressure equals atmospheric pressure.
But, the boiling point of water changes with elevation. The boiling point is a higher temperature below sea level and a lower temperature above sea level. The normal boiling point of water is 100°C (212°F, 373.1 K) at sea level. Boiling occurs when vapor pressure equals atmospheric pressure. At sea level, water boils at 100° C (212° F). At higher altitudes the temperature of the boiling point is lower. See also vaporization.
At sea level, water boils at 100° C (212° F). At higher altitudes the temperature of the boiling point is lower. See also vaporization.:max_bytes(150000):strip_icc()/BoilingWater-58dd1c2a5f9b5846837d2a23.jpg) If you want a quick and simple answer, you can say that the boiling point of water is 100 °C or 212 °F at 1 atmosphere of pressure (sea level). However, the value is not a constant.
If you want a quick and simple answer, you can say that the boiling point of water is 100 °C or 212 °F at 1 atmosphere of pressure (sea level). However, the value is not a constant.